
THE WAR ON ANTIBIOTIC
RESISTANCE
to make routine operations deadly.
Multi-drug resistance doesn't stop
SASPject from killing bacteria

IN THE WAR ON
ANTIBIOTIC RESISTANCE
to make routine operations deadly.
Multi-drug resistance doesn't stop
SASPject from killing bacteria.


FOR BACTERIA THAT'S
HARD TO RESIST
bacterial DNA: targeted bacteria
can't multiply and can't survive.

FOR BACTERIA THAT'S
HARD TO RESIST
targeted bacteria can't multiply
and can't survive.



SIMPLE DESIGN CLEVERLY
CRAFTED TO FIT EACH
TARGET BACTERIA
bacterial DNA: targeted bacteria
can't multiply and can't survive.
SIMPLE DESIGN CLEVERLY
CRAFTED TO FIT EACH
TARGET BACTERIA
from any bacteria, SASject design is bespoke for
each target bacteria, ensuring specificity with
no off-target activity.

THE BIG PICTURE
The UK government commissioned report published in 2016 by Jim O’Neill, highlights the growing problem of antimicrobial resistance (AMR). This is particularly evident for Gram negative bacteria, where resistance is now emerging to even last resort antibiotics, such as colistin. The report suggests that unless action is taken, the global burden of deaths from AMR could balloon to 10 million lives each year by 2050.

THE COMPANY
Phico Therapeutics is a biotechnology company developing a novel platform technology which it believes could form the basis for a new generation of antibiotics to overcome antibacterial resistance.
MEET THE TEAM
-
Dr Heather Fairhead FOUNDER & CEO
Dr Fairhead has over 20 years' experience directing research teams in a variety of disciplines and has led Phico in developing SASPject technology from a concept through a Phase I clinical trial. Prior to her scientific career, Heather worked in sales and marketing in a wide range of industries.
Dr Heather Fairhead FOUNDER & CEO
-
Richard Nagle CHAIRMAN
Richard Nagle is an experienced biotech executive and has built and developed business solutions across the international biopharma sector. His focus on accelerating product development has led to successful company exits or IPO, including recent CEO and Board Director of Immune Regulation (now Revolo Biotherapeutics) an innovative biotechnology platform business focused on regulating the immune system which successfully raised $50m in 2020 from Morningside Ventures. Previously, Richard served as Non-Executive Director of Peptinnovate and as CEO of a number of successful drug delivery, biotechnology and medical device businesses. He was also a Director of IMS Health and Quintiles (now IQVIA).
Richard Nagle CHAIRMAN
-
Professor Mark Wilcox NON-EXECUTIVE DIRECTOR
Mark is a Consultant Microbiologist, Head of Research and Development in Microbiology at the Leeds Teaching Hospitals (LTHT), Professor of Medical Microbiology at the University of Leeds (Leeds Institute of Molecular Medicine), and is the Lead on Clostridium difficile for Public Health England (PHE) and Medical Advisor to National Infection Prevention...
Professor Mark Wilcox NON-EXECUTIVE DIRECTOR
-
David Beadle NON-EXECUTIVE DIRECTOR
David Beadle has over 25 years' experience in the healthcare and finance industries and brings a wealth of knowledge in investment banking, finance and life sciences. David started his career working in the pharmaceutical industry for Schwarz Pharma and Schering AG in marketing and strategic development, where he was part of the global...
David Beadle NON-EXECUTIVE DIRECTOR
-
Mr Andrew Armour FINANCE DIRECTOR
Andrew is a Chartered Accountant with wide industry experience including healthcare, particularly in financial restructuring, equity fundraising and corporate acquisitions and disposals. Andrew was the Finance Director of Colchester Hospital...
Mr Andrew Armour FINANCE DIRECTOR
TECHNOLOGY
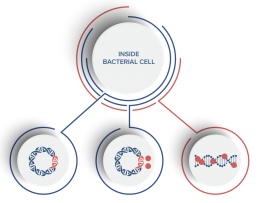
SASP gene expression system delivered to target bacteria via SASPject NDV
Bacteria read SASP gene and make SASP
SASP’s bind to and inactivate bacterial DNA
SASP gene expression system delivered to target bacteria via SASPject NDV

Bacteria read SASP gene and make SASP
SASP’s bind to and inactivate bacterial DNA

Our SASPject™ platform delivers pan-spectrum anti-bacterial proteins called small acid-soluble spore proteins, or SASPs, to selected bacterial species using engineered bacterial viruses (bacteriophages). SASPject™ works by injecting a gene that encodes SASP directly into targeted bacteria. The injected gene directs the targeted bacteria to produce SASPs, which then bind to bacterial DNA and inactivate it. SASPs “turn off” DNA so the targeted bacterial cell cannot metabolise or reproduce. The immune system can then remove the bacteria from the body.
SASPs bind to all bacterial DNA, irrespective of the sequence of that DNA. Spontaneous mutations in DNA, or the import of new DNA that gives new characteristics to the bacterial cell, are key ways in which bacteria develop resistance to antibiotics. Neither of these strategies affects the ability of SASP to bind to and inactivate bacterial DNA.
This approach has the potential to provide a number of significant advantages over traditional antibiotics:
- The unique mode of action of SASP makes it unlikely the bacteria will be able to develop resistance to this anti-bacterial protein
- SASPject technology can be used to target any selected bacteria, individual or multiple bacterial species or genera, including those that are multi-antibiotic resistant.
- Unlike conventional antibiotics, SASPject has no effect on any bacteria other than those at which it is targeted. Normal skin and gut bacteria (“good bacteria”) are unharmed.
- SASPject target specificity prevents the release of toxins and other inflammatory cell components from non-target bacteria thus potentially minimising associated side effects.
- SASPject has the potential to limit the further spread of antibiotic resistance genes and to shrink the current antibiotic resistance pool
Our SASPject™ platform delivers pan-spectrum anti-bacterial proteins called small acid-soluble spore proteins, or SASPs, to selected bacterial species using targetable nano-delivery vehicles (NDVs). SASPject™ works by injecting a gene that encodes SASP directly into the targeted bacteria.
The injected gene then produces SASPs, which bind to bacterial DNA and inactivate it. SASPs “turn off” DNA so the targeted bacterial cell cannot metabolise or reproduce. The immune system can then remove the bacteria from the body.
SASPs bind to all bacterial DNA, irrespective of the sequence of that DNA. Spontaneous mutations in DNA, or the import of new DNA that gives new characteristics to the bacterial cell, are key ways in which bacteria develop resistance to antibiotics. Neither of these strategies affects the ability of SASP to bind to and inactivate bacterial DNA.
This approach has the potential to provide a number of significant advantages over traditional antibiotics:
- The unique mode of action of SASP makes it unlikely the bacteria will be able to develop resistance to this anti-bacterial protein
- SASPject technology can be used to target any selected bacteria, individual or multiple bacterial species or genera, including those that are multi-antibiotic resistant.
- Unlike conventional antibiotics, SASPject has no effect on any bacteria other than those at which it is targeted. Normal skin and gut bacteria (“good bacteria”) are unharmed.
- SASPject target specificity prevents the release of toxins and other inflammatory cell components from non-target bacteria thus potentially minimising associated side effects.
- SASPject has the potential to limit the further spread of antibiotic resistance genes and to shrink the current antibiotic resistance pool
OUR PRODUCTS

SASPject PT3.9 is being developed against Pseudomonas aeruginosa. Whilst infections due to P. aeruginosa can involve any part of the human body, this organism is responsible for a number of hospital-acquired infections and is the third leading cause of hospital acquired pneumonia and ventilator associated pneumonia. The increasing multi-drug resistance of P. aeruginosa strains has resulted in the U.S. CDC (Centers for Disease Control and Prevention) classifying P. aeruginosa as a serious threat to human health, and the WHO classifying it in the top three bacteria needing new treatments.

SASPject™ PT4 and SASPject™ PT5 are being developed for intravenous use against both Klebsiella pneumoniae and Escherichia coli respectively. These bacteria cause a wide range of infections, which can be serious or life-threatening as isolates which are resistant to almost all conventional antibiotics continue to spread around the globe, resulting in very poor treatment outcomes.

SASPject™ PT1.2 targets Staphylococcus aureus, including MRSA. MRSA infections are now a global problem in hospitals, with thousands of fatalities recorded as a result of their presence, and their control is vital to many national health systems. SASPject™ PT1.2 will be used for the intra-nasal decolonisation of the bacteria. A Phase I clinical trial has been successfully completed.

SASPject PT3.9 is being developed against Pseudomonas aeruginosa. Whilst infections due to P. aeruginosa can involve any part of the human body, this organism is responsible for a number of hospital-acquired infections and is the third leading cause of hospital acquired pneumonia and ventilator associated pneumonia. The increasing multi-drug resistance of P. aeruginosa strains has resulted in the U.S. CDC (Centers for Disease Control and Prevention) classifying P. aeruginosa as a serious threat to human health, and the WHO classifying it in the top three bacteria needing new treatents.

SASPject™ PT4 and SASPject™ PT5 are being developed for intravenous use against both Klebsiella pneumoniae and Escherichia coli respectively. These bacteria cause a wide range of infections, which can be serious or life-threatening as isolates which are resistant to almost all conventional antibiotics continue to spread around the globe, resulting in very poor treatment outcomes.

SASPject™ PT1.2 targets Staphylococcus aureus, including MRSA. MRSA infections are now a global problem in hospitals, with thousands of fatalities recorded as a result of their presence, and their control is vital to many national health systems. SASPject™ PT1.2 will be used for the intra-nasal decolonisation of the bacteria. A Phase I clinical trial has been successfully completed.
MEDIA AND INVESTORS
Phico Therapeutics establishes Scientific Advisory Board
The SAB will provide scientific oversight and strategic advice to support the progression of Phico’s engineered phage antibacterial therapy, SASPject, through clinical trials.
01/02/2022
Appointment of Richard Nagle to the Phico Therapeutics Board
Phico Therapeutics is pleased to announce the appointment of Richard Nagle as Chairman of its Board.
28/06/2021
The Naked Scientists Interview: Little Pharma, off the beaten track
Dr Heather Fairhead, Interview with The Naked Scientists – “Little Pharma: Off the beaten track”
12/09/2017
BBC Radio Cambridgeshire – Morning Show:
Dr Heather Fairhead, Phico’s CEO, talks to Dotty McLeod about PT3
17/11/2016
Press Release:
Phico moves forward with global fight against antibiotic resistant ‘superbugs’, raising more than £3.5M to take its lead PT3 program into the clinic.
15/11/2016
Dr Heather Fairhead, Phico’s CEO, talks to Dotty McLeod on the Breaksfast Show during World Antibiotic Awareness Week 2015.
18/11/2015
Dr Heather Fairhead, Phico’s CEO, is interviewed as part of an article on antibiotic resistance, during the 2015 World Antibiotic Awareness Week.
18/11/2015
Phico Therapeutics Receives Translation Award To Advance Its SASPject™ PT4 aimed at Escherichia coli and Klebsiella pneumoniae Towards Clinical Trials
03/09/2015
Andie Harper’s Mid Morning Show, BBC Radio Cambridgeshire:
Radio Interview with Phico Therapeutics’ CEO, Dr Heather Fairhead. Heather discusses the problems we are facing with antibiotic resistant bacteria.
08/01/2015
Phico Therapeutics and its technology features in the pages of Wired Magazine.
11/01/2014
Interview with Dr Heather Fairhead, Phico’s CEO, following the call to arms for development of new antibiotics by Professor Dame Sally Davies, UK Chief Medical Officer.
03/11/2013
Phico Therapeutics CEO Heather Fairhead discusses antibiotic resistance on Sky News
15/03/2013
Engineered Bacteriophage as a Delivery Vehicle for Antibacterial Protein, SASP.
14/10/2021
Feature in Drug Discovery Today
A commentary on the development of engineered phage as therapeutics
14/10/2021
Unfortunately, we don’t have any open job vacancies at this time, but please check back soon for future opportunities.
CONTACT US
GENERAL ENQUIRIES
- +44(0)1954 741040
- info@phicotx.co.uk
- Phico Therapeutics Ltd, Bertarelli Building, Bourn Hall, High Street, Bourn, Cambridgeshire, CB23 2TN
